- Automation & Control Gear
- Cables & Wires
- Enclosures & Server Racks
- Fuses & Circuit Breakers
- HVAC, Fans & Thermal Management
- Lighting
- Relays & Signal Conditioning
- Switches
- Batteries & Chargers
- Connectors
- Displays & Optoelectronics
- ESD Control, Cleanroom & PCB Prototyping
- Passive Components
- Power Supplies & Transformers
- Raspberry Pi, Arduino, ROCK, STEM Education & Development Tools
- Semiconductors
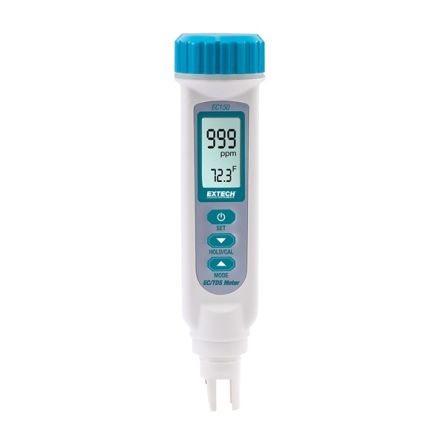
Conductivity Meters
Conductivity meters are devices for measuring the electrical conductivity in a solution. Distilled water is a poor electrical conductor and the substances or salts dissolved in the water determine how conductive the solution will be, as the number of dissolved ions increases, so does the solution's ability to carry an electrical charge. This electrical charge is what allows a conductivity meter to measure the conductance of a solution.
How do they work?
A conductivity meter consists of a probe that measures conductivity. A small electrical current flows between two electrodes set a certain distance apart, usually around 1 cm. If there is a high concentration of ions in the solution, the conductance is high, resulting in a fast current. The electrical current is slower and gives a smaller reading when a lower concentration of ions is present.
They are commonly used in hydroponics, aquaculture and freshwater systems to monitor the number of nutrients, salts or impurities in the water. Conductivity meters must be calibrated to provide accurate results.
Advantages
• Available as a controller or an analyser to be implemented in process situations
• Able to make a sampling measurement in less than one second
Disadvantages
• Cannot distinguish between different types of ions
• Conductivity meters are temperature dependent, conductance increases approximately 2% per °C
• Does not measure the number of ions in a solution directly
Uses
• Agriculture to measure the salinity levels of surface water and of soil samples
• Wastewater treatment facility to measure the quality of water
• Water conditioning (softener, demineralisation, aquariums, pollution control)